Environment & Energy
Related: About this forumEffects of reprocessed uranium multi-recycle on proliferation resistance of plutonium and uranium.
The paper I'll discuss in this post is this one: Masaki Saito and Sunil S. Chirayath, Effects of reprocessed uranium multi-recycle on proliferation resistance of plutonium and uranium Proceedings of the INMM & ESARDA Joint Annual Meeting, August 23-26 & August 30-September 1 2021, Virtual Meeting.
The paper is readily available for free on the internet, nevertheless I will excerpt it and produce some useful diagrams. It concerns, in part and in particular, the accumulation of the uranium isotope 236U which no longer occurs naturally, but can be obtained from used nuclear fuel. 236U is a "parasitic" nucleus with respect to neutrons. It is the second heaviest actinide that does not possess a critical mass, the other being 238U which represents the bulk of the Earth's billions of tons of uranium.
I made the same point the paper makes - which was certainly not original with me - some years back on another website:
On Plutonium, Nuclear War, and Nuclear Peace
The linked paper above is more scientifically rigorous than my old post years back.
From the introduction:
... To allay the fear of the diversion of Pu to nuclear weapons in the future, the basic concept of “Protected Plutonium Production (P3) by the transmutation of minor actinides (MA)” has been proposed by Saito et al. [3,4] to increase the proliferation resistance (PR) of Pu accumulated in the used nuclear fuel discharged from LWRs and fast breeder reactors (FBRs). As shown in figure 1, 238Pu present in Pu decays through spontaneous fission producing neutrons at the rate of 2.6x10 3 n/g/s to deteriorate the nuclear explosive quality of Pu. In addition, the high decay power of 238Pu (570 W/kg) makes the processes of nuclear weapon manufacturing and maintenance technologically difficult. Therefore, the PR of Pu can be improved by enhancing the 238Pu concentration. To denature Pu by enhancing its 238Pu concentration, transmutation of MA can be sought through two nuclear chains from 237Np through 238Np to 238Pu and from 241Am through 242Am and 242Cm to 238Pu as shown in figure 2...
Table 1 in the paper gives an estimate of the world fissionable and fertile actinides uranium and plutonium, and the amount of time that these material would theoretically supply the world's reactors without the need of mining (or obtaining from seawater) any of the billions of tons of naturally occurring uranium.
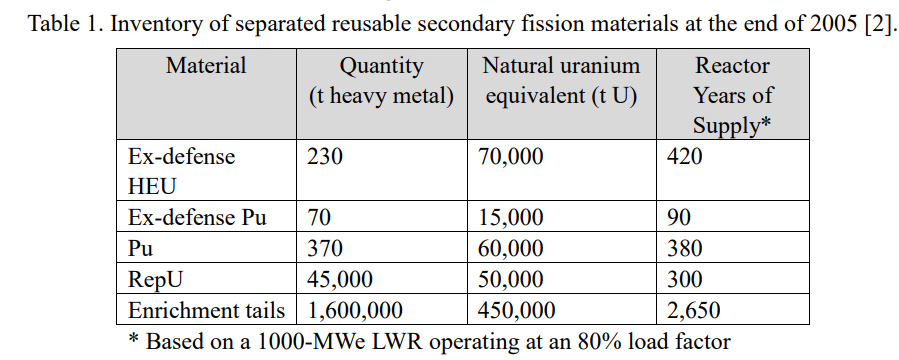
The decay heat, which, if high, makes the construction of nuclear weapons from plutonium pretty much impossible, owing to the effect of heat on the chemical explosives necessary to generate a supercritical state in plutonium unstable, as well as the spontaneous fission neutron flux from each isotope - which would cause a nuclear weapon to "fizzle" - is shown in the following figure.
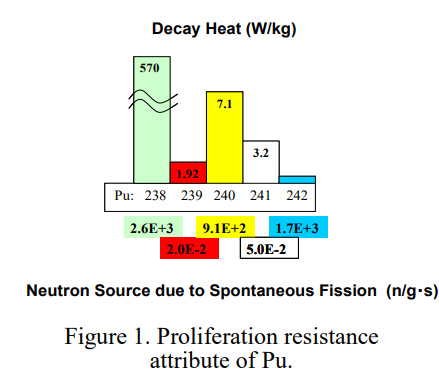
I really like the next diagram, even though it refers mainly to the properties of actinides in thermal reactors as opposed to fast reactors (in which, for example, 241Am is a potential fuel, not a burnable poison), it shows all of the readily accessible actinides that can be recovered to make clean energy up to some of the lighter curium isotopes.
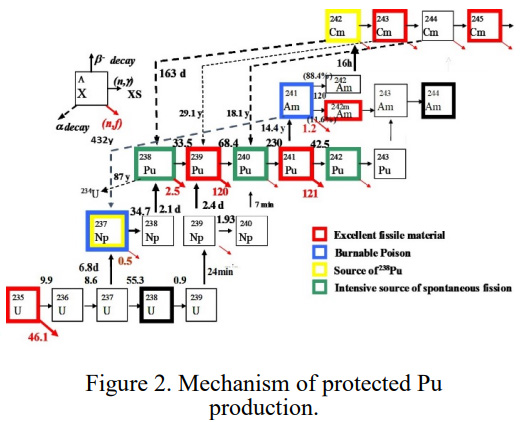
While the paper refers to the common used nuclear fuels, which generally rely on enriched uranium, the following diagram refers to the critical masses of uranium isotopes accessible from including thorium in the fuel. (A great deal of radioactive thorium has been mined and dumped as a side product of the lanthanide mining on which useless things like electric cars and wind turbines depend.) These isotopes are 232U and 233U. The former is often presented as non-proliferation advantage of thorium fuels because of the high gamma ray output of elements in its decay series, notably 208Tl, which, it is claimed, would lead to anyone trying to make a 233U nuclear weapon being killed. This might be slightly overstated, but is nonetheless a real issue.
Critical masses of the uranium isotopes:
Here's the money shot on why it is a good idea to continuously recycle uranium obtained from used nuclear fuel:
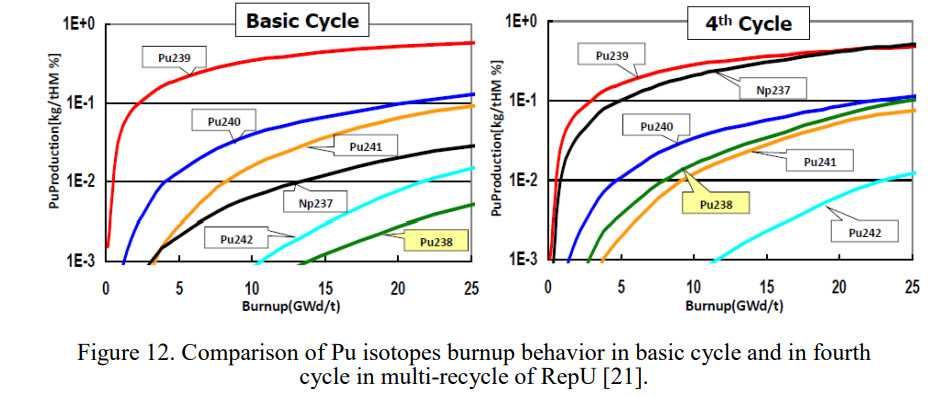
The paper refers to re-enrichment processes, of which I am not generally fond. I'm not a "thorium is the best and coolest ever nuclear fuel" kind of guy - these certainly exist - but that said, I do believe that the incorporation of thorium in "once, twice, three, times...x times through uranium" will make enrichment processes unnecessary and increase the yield of 237Np in fuel cycles, thus giving access to the important and wonderful plutonium isotope 238Pu.
Again, the full paper is available for free on the internet and interested parties are free to read it. I just wished to highlight the important points, one of which is that all of the transuranium actinides are valuable nuclear fuels that may be applied to save what is left to save (less and less each day) and possible restore that which can be restored.
Have a nice weekend. (I will, my wife and I are going art studio hopping in the catskills region this weekend.)